
Cell- and tissue culture
Cell- and tissue culture
Language:
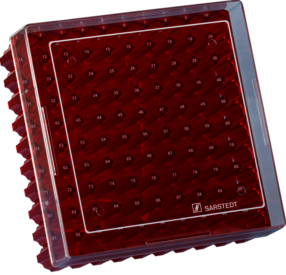
| Order number | 93.874.210 |
|---|---|
| Product description | Cryobox, with numerical coding at each aperture, for low-temperature storage, material: PC, red, slip-on lid with ventilation function, cap: transparent, (LxWxH): 132 x 132 x 53 mm, format: 10 x 10, for 100 collection tubes, for CryoPure tubes 1.2–2.0 ml internal thread, 5 piece(s)/bag |
| Graduation | no |
|---|---|
| Cap | slip-on lid with ventilation function |
| Closure type | slip-on lid |
| Design | with numerical coding at each aperture |
| Suitable for | CryoPure tubes 1.2–2.0 ml internal thread |
| Storage capacity | 100 |
| Format | 10 x 10 |
| Width of product | 132 mm |
|---|---|
| Height of product | 53 mm |
| Length of product | 132 mm |
| Product material | Polycarbonate (PC) |
|---|---|
| Colour of product | red |
| Colour of cap | transparent |
| Product category | no medical device | IVD |
|---|---|
| Batched | yes |
| Minimum order qty. | 5 |
|---|---|
| Type of smallest subpackaging | bag |
| Piece(s) / inner box | 5 |
| Piece (s) / outer case | 20 |
| Piece(s) / pallet | 720 |
| Depth of case | 135 mm |
| Width of case | 270 mm |
| Height of case | 540 mm |
| Case volume | 0.0197 cbm |
| Weight of product | 0.1593 kg |
| Weight of case | 3.71 kg |
| EAN of inner box | 4038917122894 |
| EAN case | 4038917415132 |


Because from -130°C no further biochemical reactions occur in the cells, storing these in the gas phase (< -130°C) is entirely sufficient for successful cryopreservation. We therefore recommend that CryoPure tubes are stored in the gas phase. Please note the safety advice included with our products.
Sterile
As per DIN EN ISO 11137 – 'Sterilization of health care products – Validation and routine control for sterilization with radiation'
Non-pyrogenic
Based on the LAL test as per the FDA guideline for medical devices, detection limit < 0.06 EU/ml
Non-cytotoxic
In compliance with DIN EN ISO 10993 – 'Biological evaluation of medical devices – Part 5 Tests on in-vitro cytotoxicity'
Mutagen-free
The evidence for estimating freedom from mutagens was gathered according to the Ames Test II
IVD conformity confirmed

